CONSISTENT, RELIABLE, AND LONG-ACTING T SUPPRESSION
The Vabrinty™ Delivery System Provides Consistent, Reliable, and Long-Acting T Suppression
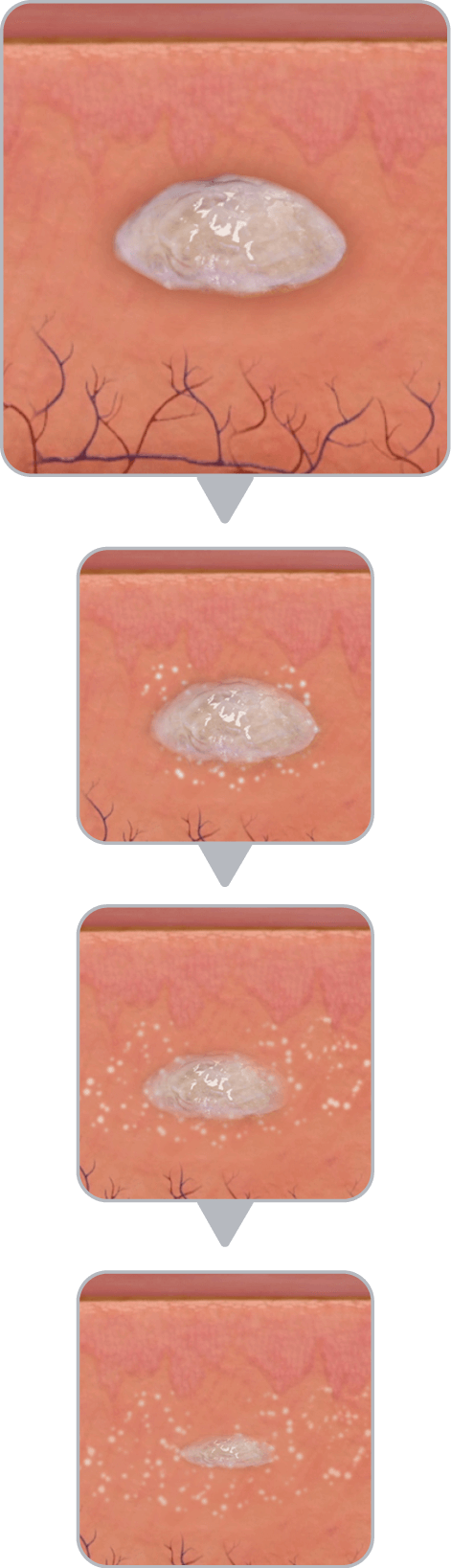
The unique in situ polymeric extended-release delivery system of Vabrinty enables sustained and controlled release of leuprolide acetate as it safely biodegrades1
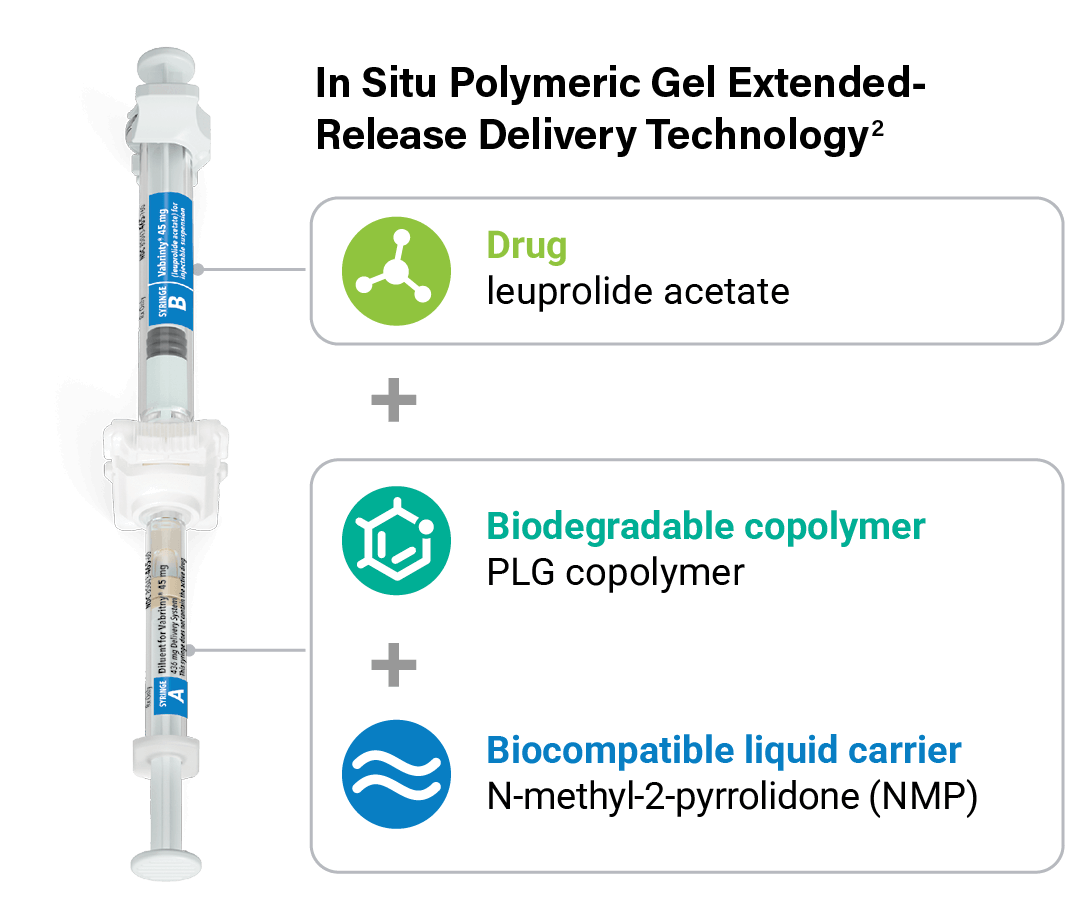
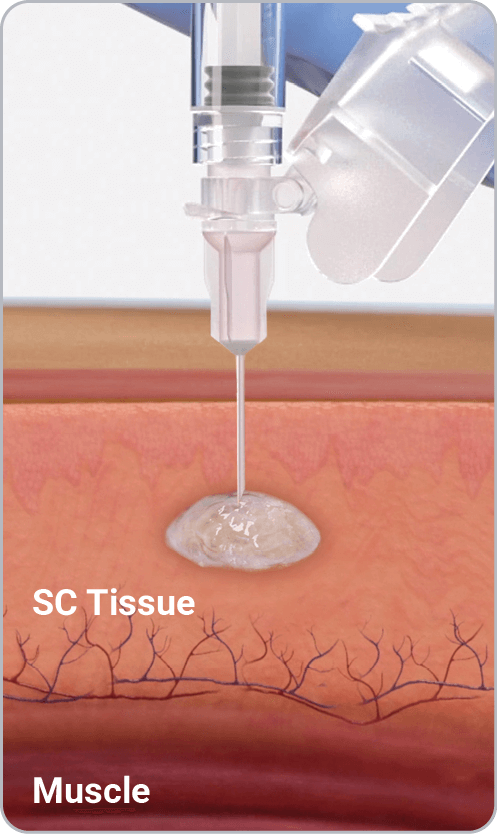
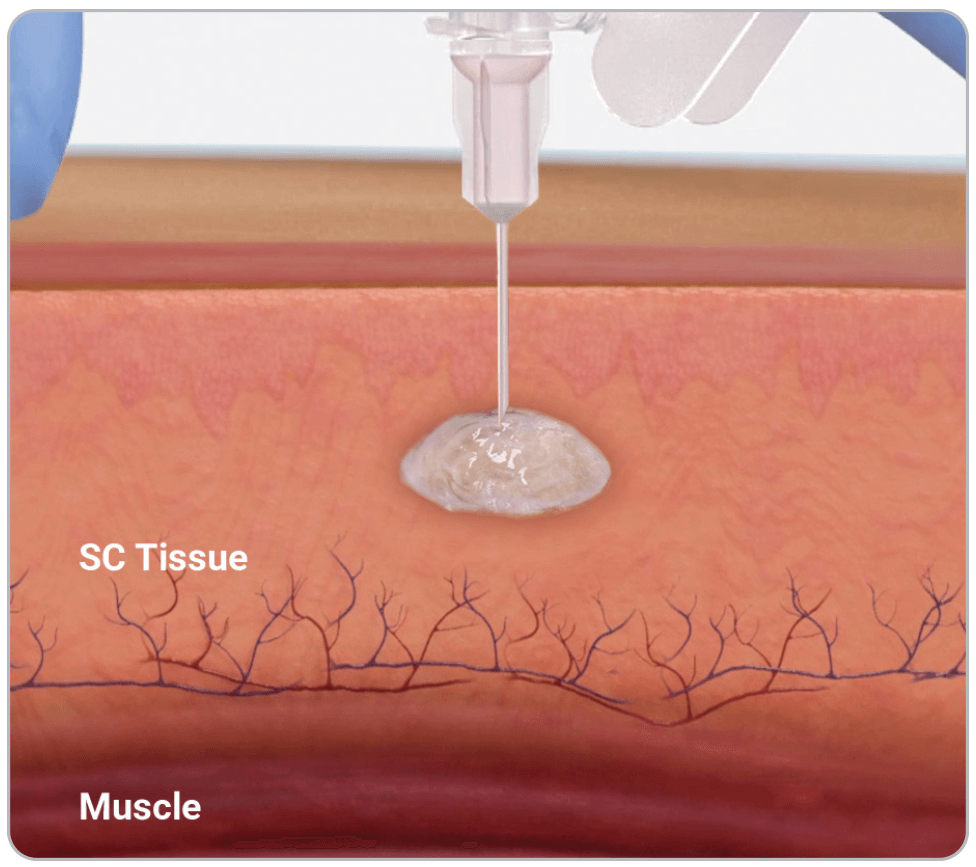
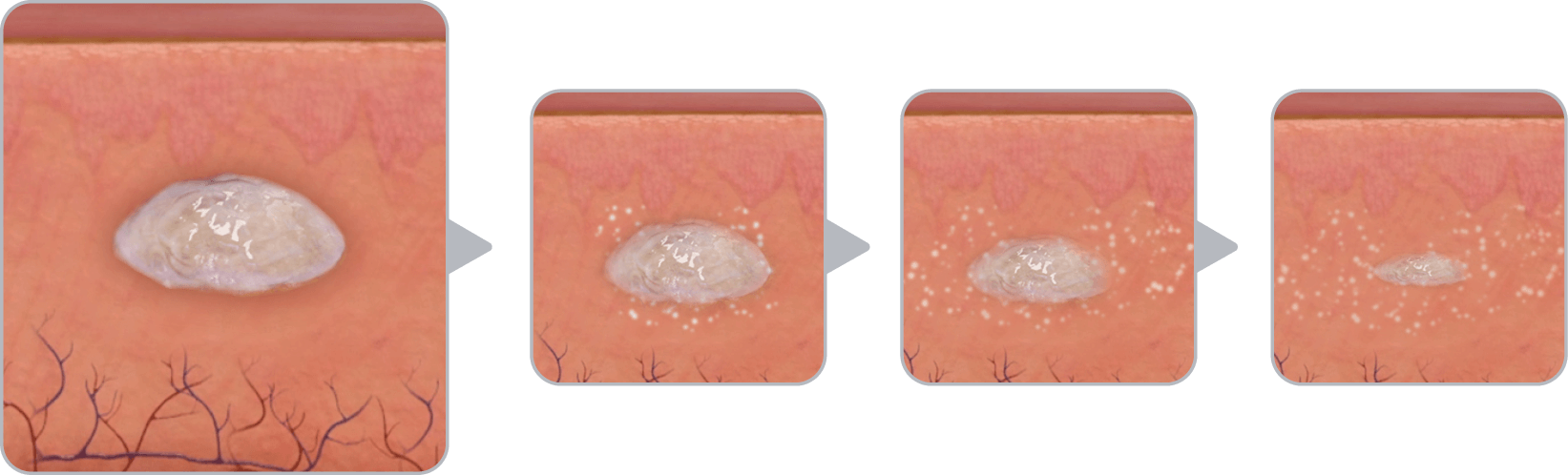
PLG copolymer solidifies in the body due to exchange of NMP and water in tissue fluid and forms a solid in situ1
T=testosterone.
Vabrinty is injected as a viscous liquid into subcutaneous tissue and remains in-situ as it slowly biodegrades over the intended dosing interval1
Prepare
If refrigerated, allow the product to reach room temperature (59 °F-86 °F) before using
Mix
Thoroughly mix Vabrinty by pushing the liquid to powder back and forth for 60 cycles between syringes to obtain a uniform suspension
Administer
Inject Vabrinty at a 90° angle into subcutaneous tissue
• Ensuring the proper injection angle enables accurate
extended-release delivery
Schedule a Mixing & Administration Training Session
Contact us at 1-844-987-6668 | info@vabrinty.com
Vabrinty™ can be left at room temperature for up to 8 weeks (56 days) prior to mixing2
30-minute administration time limit
If the product is NOT administered within 30 minutes of mixing,
it must be discarded2
• Vabrinty cannot be re-refrigerated if it has already been
mixed2,3
Storing Vabrinty
Vabrinty is a refrigerated product and should be stored at 2 °C to 8 °C (36 °F-45 °F) but can be left at room temperature for up to 8 weeks (56 days) prior to mixing and administration2
References: 1. Sartor O. Eligard®: a new form of treatment for prostate cancer. Eur Urol Open Sci. 2006;5(18):905-910. 2. Vabrinty (leuprolide acetate). Prescribing Information. UroNova, Inc. 2025. 3. Data on file. UroNova, Inc.; 2025
Important Safety Information
Vabrinty™ (leuprolide acetate) for injectable suspension is a gonadotropin releasing hormone (GnRH) agonist indicated for the treatment of advanced prostate cancer.
Vabrinty is contraindicated in patients with hypersensitivity to GnRH, GnRH agonists, or any of the components of Vabrinty.Anaphylactic reactions to synthetic GnRH or GnRH agonists have been reported in the literature. Transient increase in serum levels of testosterone during treatment may result in worsening of symptoms or onset of new signs and symptoms during the first few weeks of treatment, including bone pain, neuropathy, hematuria, bladder outlet obstruction, ureteral obstruction, or spinal cord compression. Monitor patients at risk closely and manage as appropriate.
Hyperglycemia and an increased risk of developing diabetes have been reported in men receiving GnRH agonists. Monitor blood glucose level and manage according to current clinical practice. Increased risk of myocardial infarction, sudden cardiac death and stroke has also been reported with use of GnRH agonists in men. Monitor for cardiovascular disease and manage according to current clinical practice. Androgen deprivation therapy may prolong the QT/QTc interval. Consider risks and benefits.
Convulsions have been observed in patients taking leuprolide acetate with or without a history of predisposing factors. Manage convulsions according to current clinical practice. Severe cutaneous adverse reactions (SCARs), including Stevens-Johnson syndrome/toxic epidermal necrolysis, and erythema multiforme, occurred in patients receiving Vabrinty. Monitor for and advise patients of the signs and symptoms of SCARs. May cause fetal harm.
Vabrinty may impair fertility in males of reproductive potential.
The most common injection site adverse events are transient burning and stinging, pain, bruising, and erythema. The most common systemic adverse events include mild to severe hot flashes/sweats, malaise and fatigue, weakness, myalgia, dizziness, clamminess, testicular atrophy, and gynecomastia. As with other GnRH agonists, other adverse reactions, including decreased bone density and rare cases of pituitary apoplexy have been reported.
See full Prescribing Information.
To report suspected adverse reactions, contact UroNova at 877-712-4575 or the FDA at 1-800-FDA-1088 or visit www.fda.gov/medwatch